Background:
Electrons are said to be in the ground state under stable conditions. When electrons are given energy from sources such as heat or electricity they absorb that energy and temporarily jump to a higher energy level (excited state). When they return to ground state they give off that energy in the form of electromagnetic radiation. If the electromagnetic radiation falls between 400 and 700 nanometers (nm) in wavelength, it is given off in the form of visible light.
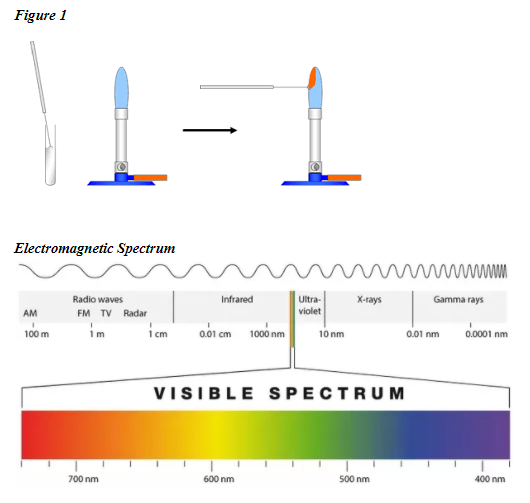
When common metal ions are heated they give off a distinct color of visible light (this is how fireworks get their color). These ions emit a unique color of light because they consist of atoms that have a unique electron configuration. Chemists can identify these elements with a flame test (see figure 1). The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. Chemists can use a spectroscope to identify these various wavelengths. The spectroscope splits light to form an emission line spectrum.


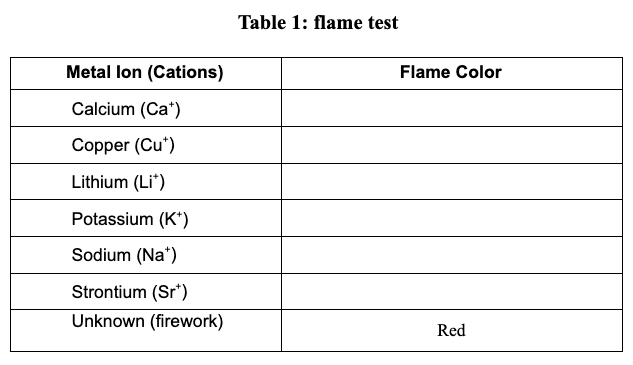
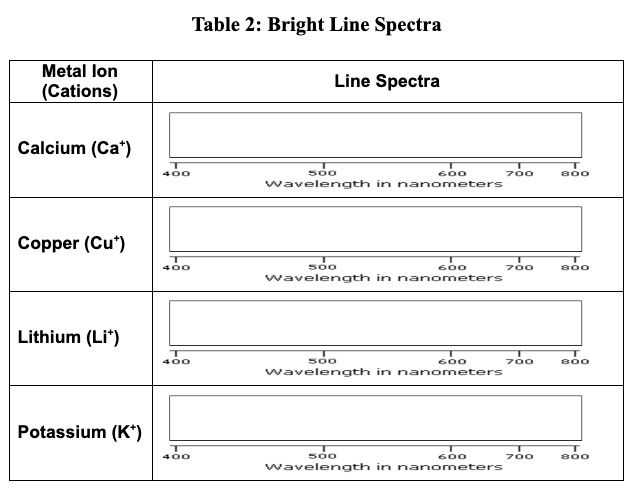
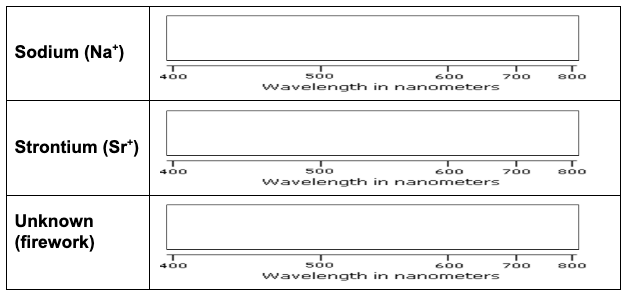
Task: Use a flame test to determine which ion salt produces the red color. Then using a spectroscope, match the bright line spectra from red fireworks to a substance in your material list. Then make your final determination of what ion in the material list causes red fireworks.
Analysis:
Based on your data, which metal ion is responsible for the red firework? Justify your answer.